Click on the titles below to navigate directly to the data opportunity:
- Aggregate Analysis of ClincalTrials.gov (AACT) Database
- Duke Cardiac Catheterization Datasets
- Pharmacokinetics of Clindamycin and Trimethoprim-Sulfamethoxazole in Infants and Children (PBPK) Datasets
- Prospective Observational Study of the Risk Factors for Hospital-Acquired and Ventilator-Associated Bacterial Pheumonia (PROPHETIC) shared thru Vivli by CTTI
- Safety, Pharmacokinetics (PK) and Hemodynamic Effects of Ambrisentan in Single Ventricle Pediatric Patients (NCT02080637)
- TOMMORROW 301 Clinical Trial Dataset
Aggregate Analysis of ClinicalTrials.gov (AACT) Database
The Clinical Trials Transformation Initiative (CTTI) created the database for the Aggregate Analysis of ClinicalTrials.gov (AACT) to facilitate research of all, or groups of, studies registered on ClinicalTrials.gov. This publicly available relational database, updated daily with content from ClinicalTrials.gov, has been used for numerous publications to characterize the clinical trials landscape, report on the state of research in specific disease areas, and investigate compliance with reporting requirements.
The AACT database can be accessed directly via the cloud and static copies can be downloaded. Curated datasets from select projects and publications are also available to allow others interested in similar topics to benefit and build upon the previous work.
- AACT Database – Connect Directly
- AACT Database – Download
- AACT Schema, Data Dictionary, Researcher’s Guide
- AACT Projects Data Sharing
Source code for AACT is available via GitHub.
AACT was upgraded in 2017 to its current version. Historical AACT database extracts from 2010-2016 are also available.
Duke Cardiac Catheterization Datasets
The Duke Databank for Cardiovascular Disease (DDCD) is a clinical care database, established in the 1960s by a research team within the Duke Division of Cardiology. As a component of the DDCD, data were collected on patients undergoing cardiac catheterization for suspected coronary artery or valvular heart disease, and on patients undergoing cardiac surgery. These data were used to generate reports for the patients’ medical records, but were also made available for clinical research, for example, research studies to improve the medical care of patients with coronary artery disease. The Duke-owned DDCD is one of the largest and oldest cardiovascular databanks in the world. Duke researchers have conducted several cardiology outcome studies using this source data.
Duke Cardiac Catheterization Research Dataset (DukeCath)
The DukeCath analysis dataset, extracted from the DDCD, includes records and variables for adult patients undergoing cardiac catheterization procedures at Duke between 1985 and 2013. This dataset has been de-identified in order to remove individually identifiable protected health information. The DukeCath dataset is suitable for clinical and/or methodological research and publications purposes. It contains one record per catheterization procedure and includes patient characteristics, catheterization results, interventional treatments, and long-term outcomes data that allow for assessments of relationships of variables with outcomes, as well as examination of time trends. The dataset includes more than 150,000 catheterization procedures in more than 80,000 unique patients.
Requestors are strongly encouraged to review the data dictionary and user documentation to make sure the relevant variables and population needed for their research are included.
Users whose proposals are successfully approved by a review committee will use this data within a secured platform, equipped with statistical and visual analytics tools. Thus far, we share this dataset through SAS, AHA, and Vivli platforms. This dataset has been approved for sharing by Duke University Health System and the Institutional Review Board.
Duke Cardiac Catheterization Educational Dataset (DukeCathR)
The DukeCath educational dataset, a de-identified and anonymized dataset extracted from the DDCD, has been intentionally modified such that any meaningful or publishable clinical interpretations are impossible. DukeCathR provides instructors and students exposure to the "real world" data for instructional analyses. The goal is to provide instructors with a resource to help teach students how to approach statistical analyses using real patient data. Any teacher, instructor, or student can request this dataset for educational purposes.
This dataset has been approved for sharing by Duke University Health System and the Institutional Review Board.
Pharmacokinetics of Clindamycin and Trimethoprim-Sulfamethoxazole in Infants and Children (PBPK) Datasets
Children go through many developmental changes during childhood, and these changes in their physiology need to be accounted for when determining accurate pediatric dosing. If developmental changes are not factored into dosing decisions, drug efficacy and safety could be negatively impacted. A new study seeks to evaluate a platform to validate a type of modeling that could be valuable in identifying the most accurate dosages during pediatric clinical trials.
Population physiologically-based (PBPK) models account for many different types of information, such as drug-specific factors like protein binding, systems-specific factors like blood flow, and other factors like genetic variants. All this information taken together can help investigators determine the dose-exposure relationship of drugs and how that changes as children grow up.
PBPK models could alleviate the burden of pediatric clinical trials by requiring fewer children for each trial while maximizing dose-based safety and efficacy. The current study seeks to evaluate a platform to validate the use of PBPK models in pediatric clinical trials. The study will test clindamycin and Bactrim (also known as TMP-SMX), which are among the most commonly used drugs to treat gram-positive infections in infants and children. Each drug is an ideal candidate for PBPK model evaluation because of their differing physico-chemical properties and elimination pathways.
The raw datasets include records collected from this trial (ClinicalTrials.gov Identifier: NCT02475876) on all of the 51 patients enrolled, ranging in age from approximately 3 months – 16 years old. Data has been de-identified to remove individually identifiable protected health information (PHI), and can be used for clinical and/or methodological research/publication. The datasets contain information which sites originally entered into the electronic Case Report Form (eCRF) during the study, or raw pharmacokinetic (PK) data entered by the lab running analysis on blood or urine samples. Depending upon the specific dataset, dataset structure may be one record per subject or multiple records per subject. Datasets provide information on adverse events, labs, concomitant medications, demographics, drug dosing, patient disposition, inclusion/exclusion criteria, medical history, PK data, and standard of care microbiological evaluations. Relationships between concomitant medications, demographics, drug dosing, PK levels, and any other data of interest can be assessed by linking records via de-identified subject number. The datasets include over 350 PK samples.
Prospective Observational Study of the Risk Factors for Hospital-Acquired and Ventilator-Associated Bacterial Pneumonia (PROPHETIC) shared thru Vivli by CTTI
The purpose of this study is to better define the intensive care unit population at highest risk for developing Hospital-Acquired and Ventilator-Associated Bacterial Pneumonia (HABP/VABP).
- View information about this study on ClinicalTrials.gov
- View information about this study on Vivli
- View the protocol from CTTI
Related Publications:
PROPHETIC: Prospective Identification of Pneumonia in Hospitalized Patients in the ICU
Safety, Pharmacokinetics (PK) and Hemodynamic Effects of Ambrisentan in Single Ventricle Pediatric Patients (NCT02080637)
The purpose of this study was to evaluate the pharmacokinetics, bioavailability and hemodynamic efficacy of ambrisentan after Fontan surgical palliation of single ventricle heart defects. Study activities and population group: Children undergoing Fontan surgical palliation for single ventricle defects will be eligible for the study. Up to 20 subjects will be enrolled (16 ambrisentan, 4 placebo) and will receive 3 days (3 doses) of ambrisentan starting on post-operative day #1 upon returning from the operating room. Ambrisentan plasma levels will be obtained at specified time points during treatment. Post-operative monitoring lines will be used to measure effects of ambrisentan on hemodynamics and pulmonary / systemic endothelial function.
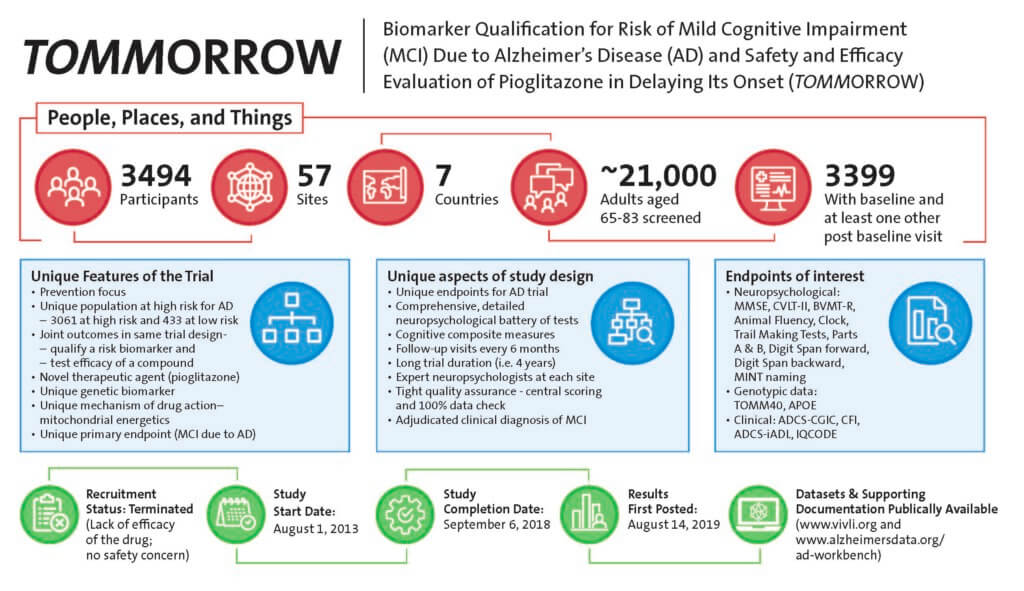
TOMMORROW 301 Clinical Trial Dataset
The Takeda TOMMORROW trial (NCT01931566) investigated a genetic-based biomarker risk assignment algorithm (BRAA) and evaluated the safety and efficacy of investigational drug pioglitazone 0.8 mg SR in delaying the onset of Mild Cognitive Impairment (MCI) due to AD in cognitively normal individuals projected to be at high risk, as determined by the BRAA. The trial was discontinued based on interim analysis. The trial was one of the first secondary prevention studies with Alzheimer’s disease. As such, it used novel cognitive endpoints and operationalized the primary endpoint, MCI due to AD. Duke University faculty provided some of the scientific thought leadership in the Takeda TOMMORROW clinical trial. Duke received the anonymized SDTM, ADaM and Imaging datasets from Takeda. Duke collaborated with Gates Alzheimer’s Data Discovery Initiative (ADDI) which is dedicated to enhance the usability and interoperability of Alzheimer’s disease datasets to develop a User Guide containing commonly used algorithms, derived variables, composites aimed to help investigators navigate the complex datasets. Duke also collaborated with the Global Alzheimer’s Association Interactive Network (GAAIN) to provide potential users of the TOMMORROW trial data exploratory views of the data elements preparatory to research.
- ADDI Work Bench (User Guide and Navigation Tools)
- GAAIN Interrogator (Exploratory tool, reviews preparatory to research)
- Clinicaltrials.gov (NCT01931566) (Clinical Trials.gov records)
Duke investigators should contact Dr. Kathleen Welsh-Bohmer. All others can click the button below.

The Duke Clinical Research Institute is a member of Vivli, an independent, non-profit organization that has developed a global data-sharing and analytics platform. Click here to learn more about Vivli.