The DCRI utilizes the full range of services provided by the Duke Early Phase Clinical Research Unit (DEPRU) in order to accelerate the availability of therapies, diagnostics, and medical devices to humans. DEPRU, part of the Duke Office of Clinical Research, works with a variety of sponsors and partners to conduct a broad range of early phase studies.
The Path to Proof
With 50+ experienced clinical research professionals, a state-of-the-art research unit, and robust data analytics, DEPRU offers a different approach to early phase research, including:
- Thought leadership and scientific insight in early phase clinical research that leads to innovation
- Full-service capabilities and customized study design grounded in the reality of clinical care
- Access to healthy and diverse disease-specific patient populations
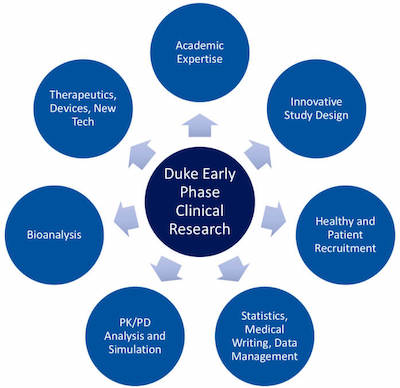
Capabilities
By leveraging the unique capabilities of an academic medical center, the Duke Early Phase Clinical Research Unit has the expertise and resources to conduct a variety of studies, from proof-of-concept to first-in-human.
The following capabilities are available:
- Comprehensive Early Phase Research Services
- Specialized Facilities and Technologies
- Rapid Recruitment
- Study Design
- Data Analytics
Learn More
Learn about the Duke Early Phase Clinical Research Unit's available studies and volunteer registry by visiting the Duke Office of Clinical Research website. Driving directions and contact information are also available.